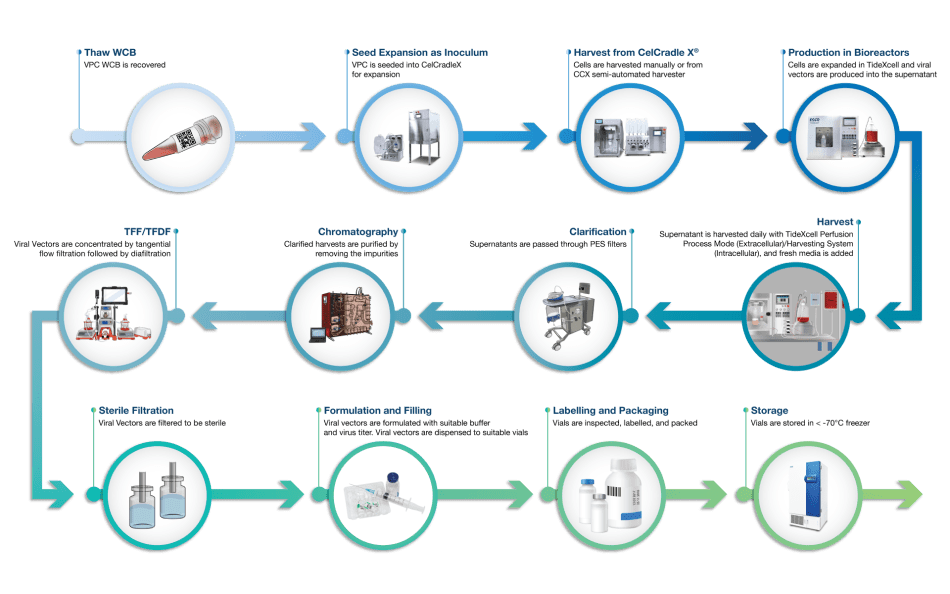
Our Manufacturing Workflow
Our services establish an optimal viral vector production process using the innovative Tide Motion technology to produce high yield and high-quality viral vectors. The optimized lab-scale process will be linearly scaled up to the manufacturing scale per the requirement of the clients.